Good Laboratory Practices:
- Maintain good housekeeping and tidiness.
- Keep all aisles and exits clear of obstacles.
- Reduce all tripping, slipping, and fall hazards.
- Clean all workspaces within a reasonable amount of time after work is finished.
- Label all containers with chemical content and responsible person’s name.
- Know where spill kits are available.
- Know evacuation routes.
- Know where emergency contact numbers are posted.
- Have reactive chemicals properly stored and well labeled.
- Have appropriate personal protection equipment (PPE) available and in good condition.
- Have Material Safety Data Sheets (MSDSs) and other safety information readily on hand.

Chemical Hygiene
Many chemical are routinely handled in the laboratory. The hazardous nature of a chemical depends not only on what it is, but also on how it is handled. In determining the hazardous nature of a chemical, consider the characteristics of the chemical. Most chemicals fall into one of four categories:
- Ignitable – a chemical with a flashpoint of less than 140 degress F.
- Corrosive – a chemical with pH less than 2 or greater than 12.5 (i.e., strong acid and bases).
- Reactive – a chemical that is explosive, shock sensitive, reacts to produce heat, light or toxic products or combines violently with air or water.
- Toxic – a chemical that has an adverse effect on organisms in relatively low doses or small quantities.
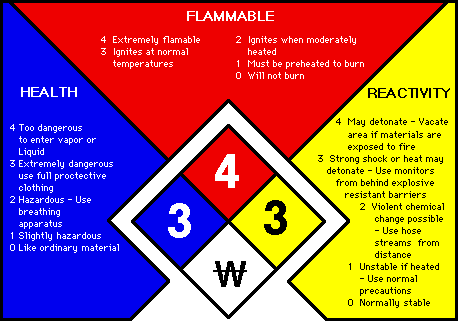

The National Fire Protection Association (NFPA) system utilizes a diamond diagram, divided into four color-coded sections:
- Blue – health hazard
- Red – fire hazard
- Yellow – reactivity hazard
- White – other hazard
Within each section, a number ranks the degree of hazard:
- 4 – extreme hazard
- 3 – serious hazard
- 2 – moderate hazard
- 1 – slight hazard
- 0 – no or minimal hazard
The white section alerts the user to special hazards the material may possess.
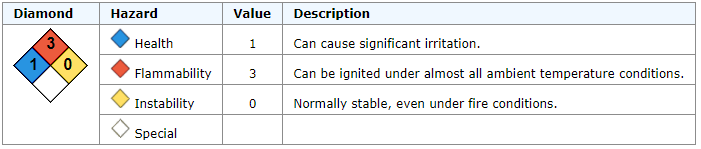

Methanol, CAS No. 67-56-1
Flammable.
Can cause significant irritation.
Carbon Tetrachloride, CAS No. 56-23-5
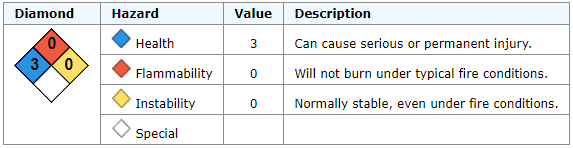
Nitric Acid, CAS No. 7697-37-2
Corrosive. May ignite combustibles. May react on contact with water.Mixing Chemicals
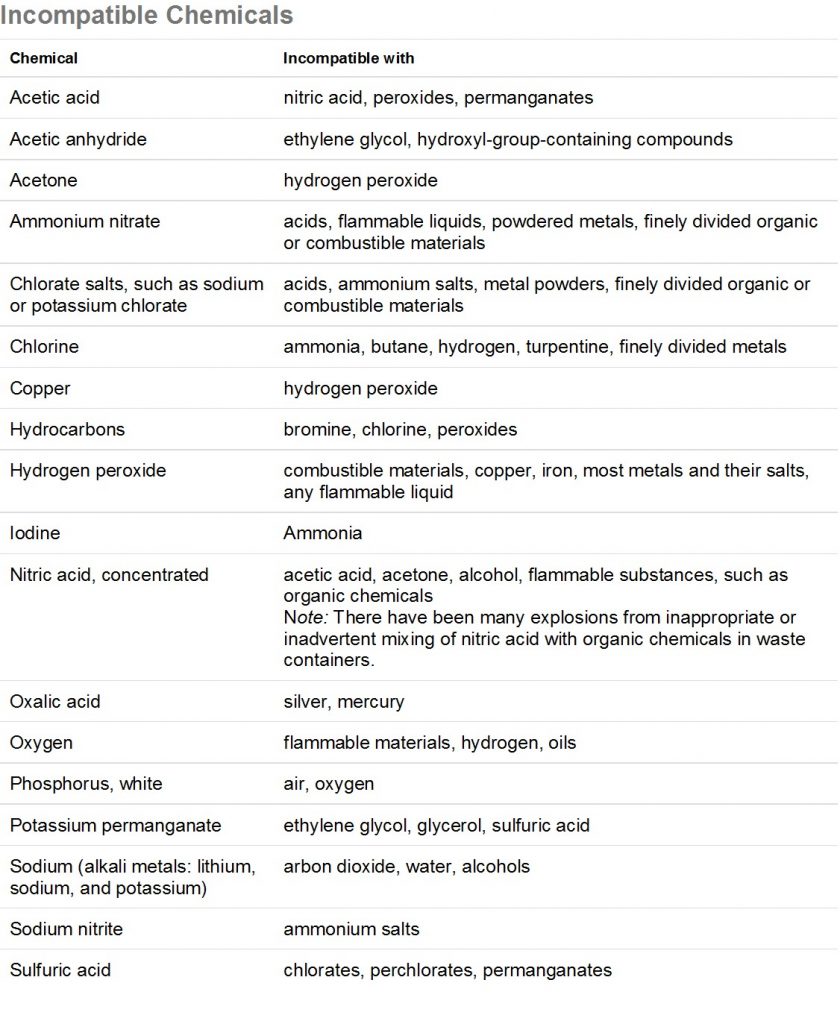
Mixing of chemicals can lead to dangerous conditions such as the release of toxic gases, release of flammable gases, violent heating and splattering, or explosive reactions. Before mixing any chemicals, refer to a chemical incompatibility list (such as the one below) and the chemicals’ MSDS’s. Some examples that could occur with chemicals common to the ERC are the mixing of nitric acid and methanol waste, which will result in an explosive mixture, or adding water to concentrated acid, which is very exothermic and will result in splattering of the corrosive acid. Do not mix any chemicals unless you know it is safe! Be aware of what is in a waste bottle before disposing of your waste and label the waste properly.

Storing Chemicals
In general, dry reagents, liquids and compressed gases should be stored separately, then by hazard class.
Segregate dry reagents as follows:
- Oxidizing Salts
- Flammable Solids
- Water-Reactive Solids
- All Other Solids
Segregate liquids as follows:
- Acids
- Bases
- Oxidizers
- Perchlorates
- Flammable or Combustible Liquids
- All Other Liquids
Storing Flammable Chemicals
- Quantities should be limited to the amount necessary for the work in progress.
- No more than 10 gallons of flammable and combustible liquids, combined, should be stored outside of a flammable storage cabinet unless safety cans are used. When safety cans are used, up to 25 gallons may be stored without using a flammable storage cabinet.
- Storage of flammable liquids must not obstruct any exit.
- Flammable liquids should be stored separately from strong oxidizers, shielded from direct sunlight, and away from heat sources.
Working with Flammable Chemicals
- Control all ignition sources in areas where flammable liquids are used. Smoking, open flames, and spark-producing equipment should not be used.
- Whenever possible, use plastic or metal containers or safety cans.
- When working with open containers, use a fume hood to control the accumulation of flammable vapor.
- Use bottle carriers for transporting glass containers.
- Use equipment with spark-free, intrinsically safe induction motors or air motors to avoid producing sparks. These motors must meet National Electric Safety Code (US DOC, 1993) Class 1, Division 2, Group C-D explosion resistance specifications. Many stirrers, Variacs, outlet strips, ovens, heat tape, hot plates and heat guns do not conform to these code requirements.
- Avoid using equipment with series-wound motors, since they are likely to produce sparks.
- Do not heat flammable liquids with an open flame. Steam baths, salt and sand baths, oil and wax baths, heating mantles, and hot air or nitrogen baths are preferable.
- Minimize the production of vapors and the associated risk of ignition by flashback. Vapors from flammable liquids are denser than air and tend to sink to the floor level where they can spread over a large area.
- Electrically bond metal containers when transferring flammable liquids from one to another. Bonding can be direct, as a wire attached to both containers, or indirect, as through a common ground system.
- When grounding non-metallic containers, contact must be made directly to the liquid, rather than to the container.
- In the rare circumstance that static cannot be avoided, proceed slowly to give the charge time to disperse or conduct the procedure in an inert atmosphere.
Fume Hood Safety
The health and safety of laboratory personnel and building occupants must be the primary goal of laboratory management. Properly functioning fume hoods help achieve this goal with respect to the hazards of chemical vapors and other harmful airborne substances. It is important to remember that a fume hood is not a storage area. Keeping unnecessary equipment and chemicals in the hood may cause airflow blockage. Here are a few health and safety tips concerning fume hoods:

- Substitute toxic chemicals with less hazardous materials whenever possible.
- Keep fume hood exhaust fans on at all times.
- Perform all work six inches inside the hood.
- Never place your head inside the hood.
- Keep the hood sash closed as much as possible at all times to reduce face velocity and energy.
- Keep lab doors closed to ensure negative room pressure to the corridor and proper air flow into the hood.
- Keep the slots of the baffle free from obstruction.
- Do not use the hood as a waste disposal mechanism (e.g., for evaporation of chemicals).
- Avoid rapid movements in front of or inside the hood, including opening and closing the fume hood sash rapidly and swift arm and body movements. These actions may increase turbulence and reduce the effectiveness of fume hood containment.
- Do not override or disable mechanical stops on the sash.
- Have a general awareness of the operation of the hood, and be aware of any differences in visual or audible cues that may imply a change in function.
- Make sure that equipment, heating mantles, or laboratory kits are not pushed all the way back in the hood. This situation tends to hinder the flow of the more dense vapors from being expelled from the hood.
- Do not use the laboratory fume hoods as a chemical storage cabinet. If a hood contains a large quantity of bottled chemicals, it is time to do some housekeeping and return the chemicals to the chemical storeroom or the hazardous waste storage (whichever is appropriate).
Chemical Spills
Chemical spills are to be cleaned up immediately using the proper procedure. Safety goggles, gloves, and a lab coat should be worn during a spill clean up.
Spilled Liquids – acids, bases, and organic solvents. Specialty spill kits are used to absorb 0.5-1.0 liter. These are located on the shelf adjacent to the stockroom computer. There are large boxes of absorbent located in the bottom of the reactive chemical cabinet to be used on larger spills. One should make a dike around the spill to contain it and then use more of the absorbent inside the absorbent dike to complete the absorption process. It is essential that the hoods in the laboratory affected by the spill be turned on to reduce the amount of vapors remaining in the air in this room. If the chemical is toxic or vapors are filling the room (even if only a small amount of the chemical has been spilled out in the lab proper), the laboratory shall be evacuated. This does not necessarily mean that the entire building must evacuate.
Waste
If you have chemical waste to dispose, ask someone in the lab who is certified to dispose of waste which waste receptacle should be used. Be aware of the chemical compatibility chart. It is best to neutralize acid and bases in a large amount of water. Never add water to a strong acid or strong base, as it will rapidly heat and sputter. It is important to label your waste and record anything that goes into a waste receptacle.
- Never dispose of liquid waste in the dumpster.
- Sharps must be placed in sealed, crush-proof containers prior to disposal.
- Non-hazardous solids may be disposed in the dumpster.
- Hazardous waste must be disposed through Environmental Health Services (EHS).
Use of Compressed Gases
Compressed gases can become dangerous projectiles if they fall and compromise safety.
Storage of Compressed Gas Cylinders
Compressed gas cylinders should be stored with a secure, approved strap or chain. Always keep covers on compressed gas cylinders when not in use.
Moving Gas Cylinders
To move gas cylinders, secure the cylinder in the tank dolly using the strap and make sure the cap is tightly fastened.